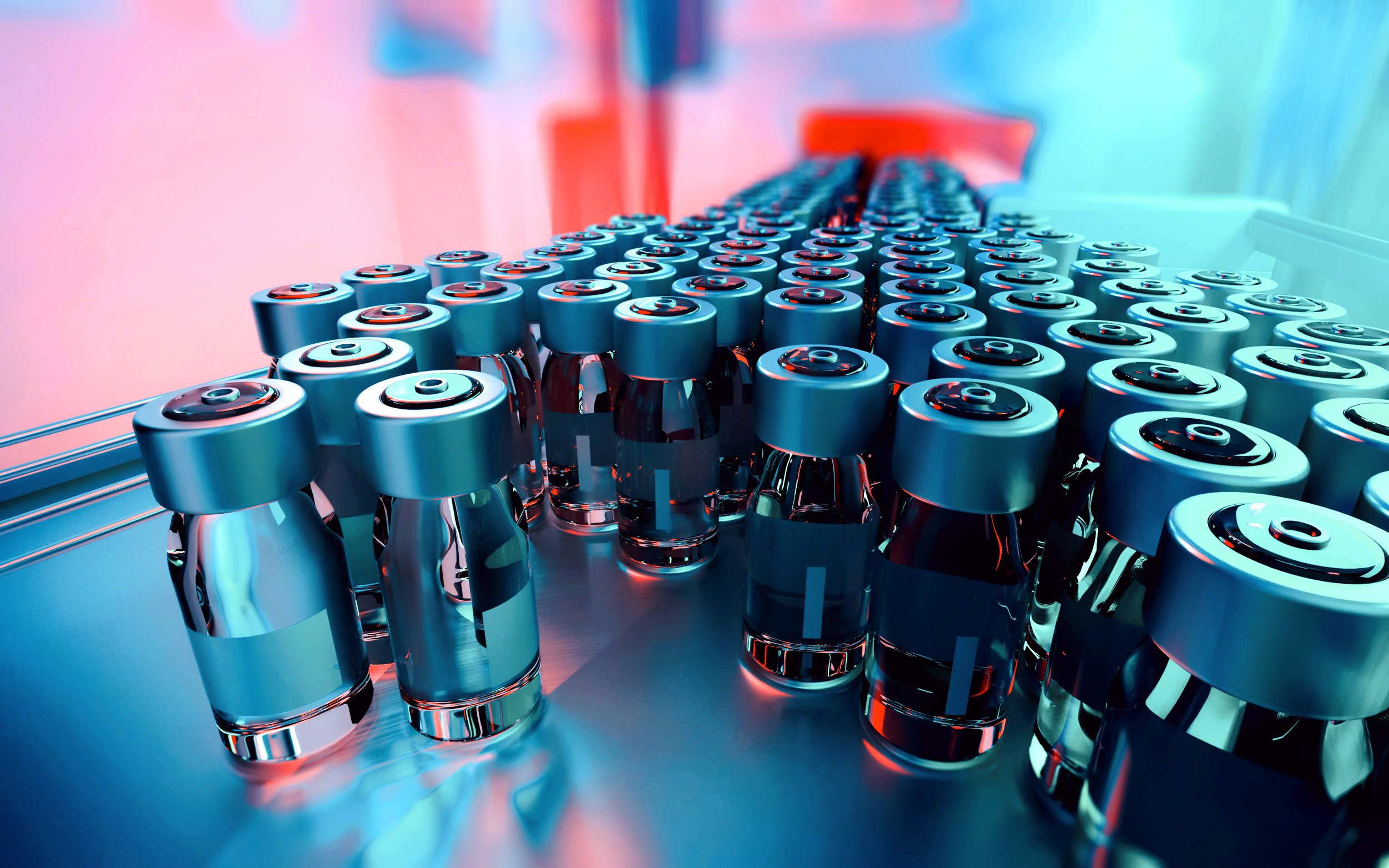
Ensuring the integrity, compliance, and timely delivery of your clinical drug products is paramount to the success of your trials. At NORCAL GMP, we offer a comprehensive, integrated solution for all aspects of clinical drug product labeling, packaging, storage, and distribution. Our robust systems, experienced team, and cGMP-compliant facilities are designed to streamline your clinical supply chain, maintain product quality, and ensure full traceability from our site to clinical sites nationwide and worldwide.
Our Comprehensive Clinical Drug Product Services:
We provide a seamless, regulated pathway for your clinical materials, from meticulous preparation to final delivery:
Clinical Drug Product Labeling and Distribution
-
Regulated label design for vials and outer packaging, ensuring full compliance with clinical trial guidelines.
Access to customizable templates and advanced application tools for precise label production.
Rigorous label inspection procedures to verify accuracy and quality before application.
Proven experience with label design and product labeling, with 12 lots completed across 3 clinical programs.
-
Final packaging solutions tailored for clinical trial materials.
Features include tamper-evident packaging to ensure product security.
Comprehensive batch tracking capabilities integrated into the packaging process.
-
Monitored and regulated drug product storage with precise temperature monitoring and diligent expiration date management.
Extensive cold storage options including 2-8°C refrigerators, -20°C freezers, and a dedicated -80°C freezer farm.
A specialized walk-in cold room featuring dedicated cages for each drug product, with capacity for storage and monitoring of over a million vials.
All storage is supported by backup generator and UPS systems to ensure continuous operation.
-
Qualified and trackable worldwide drug product distribution capabilities.
Validated temperature-controlled shipments to maintain product integrity during transit.
Integration with advanced logistics systems, enabling real-time monitoring of shipments.
Comprehensive documentation provided for compliance and traceability purposes.
Experienced in depot management, serving 20 qualified clinical sites across the U.S.A., with over 100 shipments processed nationwide.
Experience supporting multiple clinical programs including OLE (15 sites), Stroke (5 sites), EAP (105 Providers), and Parkinson’s Disease (6 active sites).


